


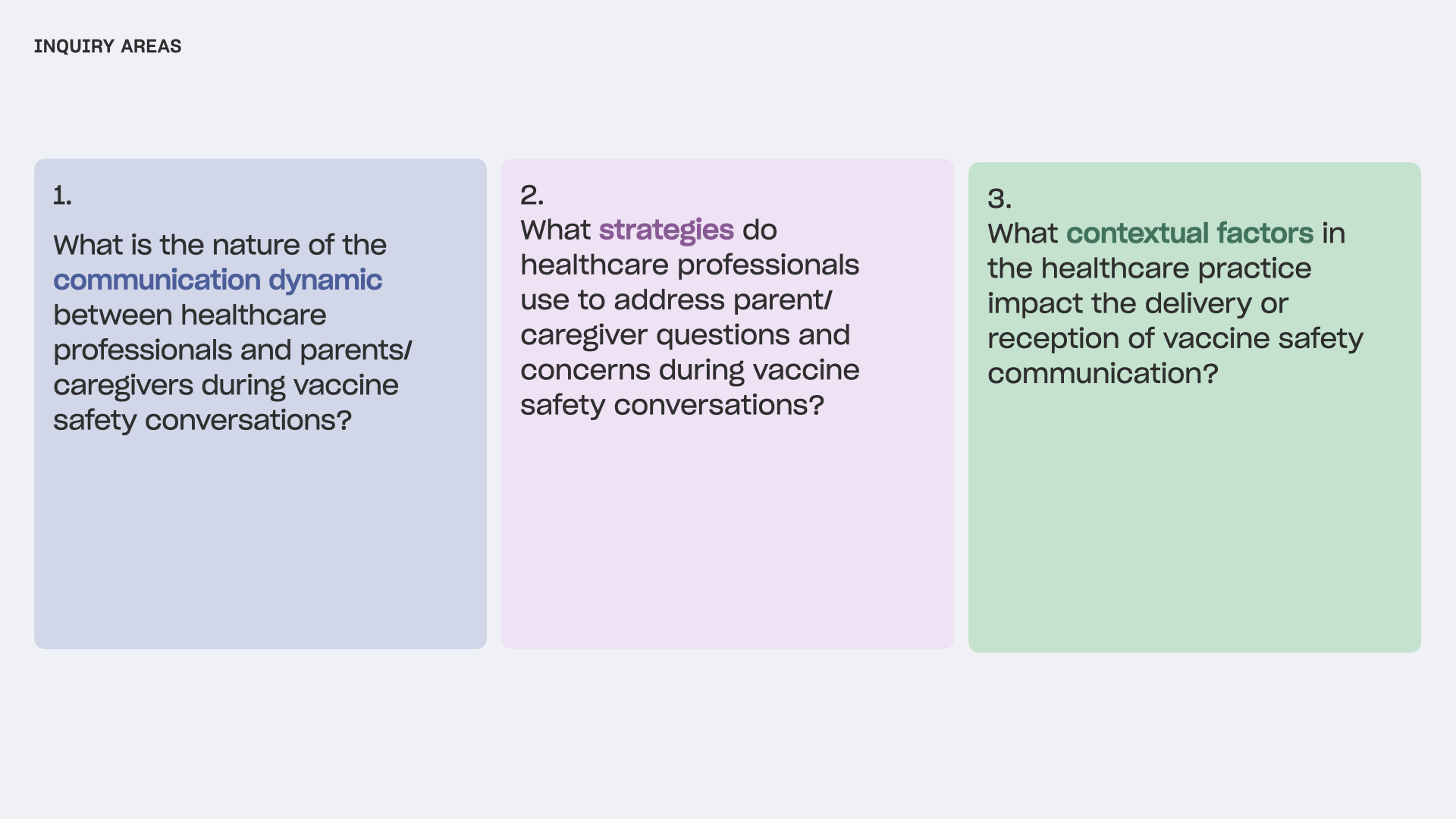
In clinics across the country, healthcare workers communicate daily with parents and caregivers about vaccine safety, shaping decisions around their children’s vaccination. To better prepare these workers to navigate vaccination conversations, it is crucial to understand the nature of these interactions and the most effective approaches.
The Research Triangle Institute International (RTI) led a year-long effort on behalf of the US Centers for Disease Control and Prevention (CDC) to understand the dynamics influencing how childhood vaccine safety information is communicated and accepted within healthcare environments. The Public Policy Lab (PPL) partnered with RTI to conduct observational research, focusing on the interactions between parents/caregivers and healthcare professionals in healthcare settings across the United States, in support of RTI’s larger research objectives.
The findings from this research support national initiatives to improve vaccine safety communication and advance the breadth of knowledge available regarding vaccine safety communication.
Disclaimer
This project is supported by the Centers for Disease Control and Prevention (CDC) of the U.S. Department of Health and Human Services as part of a project award totaling $2,770,000 with 100 percent funded by CDC/HHS. The contents of this website are those of the author(s) and so not necessarily represent the official view of, nor an endorsement, by CDC/HHS, or the U.S. government.
Appointments Observed
Interviews Conducted
Over the course of two months, the PPL team conducted an observational study on vaccine safety communication dynamics between pediatric healthcare professionals and parents/caregivers across the country.
We conducted the study on-site at eight healthcare practice locations across the United States, speaking with healthcare professionals, healthcare practice staff, and parents and caregivers. The healthcare settings ranged from independent pediatric practices to hospital-affiliated pediatric clinics. We collected firsthand data from participants using a variety of methods, including the observation of patient appointments, walkthroughs of clinics, semi-structured interviews, and a survey.
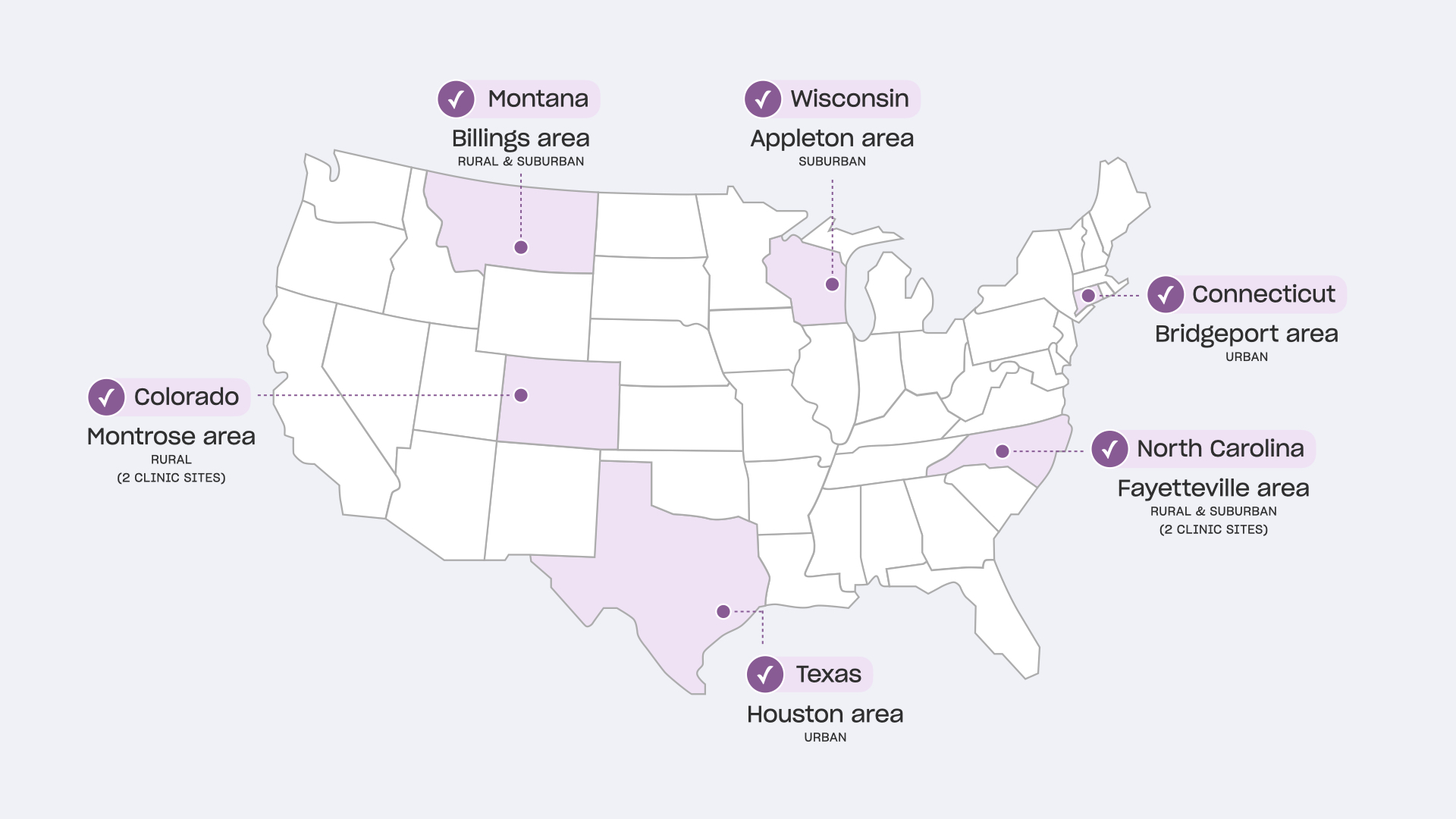
Research Locations
We prioritized practices with higher levels of vaccine concerns, diversity of patient population served, varying types of organizational structures, geographic locations, and population density levels.
Research Methods
Clinic Walkthroughs
We followed healthcare providers as they moved through their practice, including waiting rooms, exam rooms, and nurses’ stations. We asked them to explain their activities in each space and how they interact with the physical environment. If any vaccine-related materials were visible, we asked them to explain how staff and families interacted with them.
Vaccine Material Collection
At all clinics, we collected vaccine communication materials like vaccine information sheets, brochures, vaccination schedules, and other analog materials that aided families in understanding the vaccines their child might be receiving. Some practices also shared resources they provide to parents/caregivers who have vaccine hesitancy or concerns.
Survey
We administered a survey to participating parents/caregivers before their child’s appointment to gain a baseline understanding of their attitudes toward vaccines and vaccination.
Observation
With consent, we conducted contextual observations of interactions in exam rooms, waiting rooms, hallways, and clinical team workspaces. We recorded and observed live-streamed video of routine pediatric appointments with special attention to interactions related to vaccination. We did not observe the actual administration of vaccinations by nurses, which happened after the clinical conversations had ended.
Semi-Structured Interviews
We conducted semi-structured interviews with parents/caregivers and healthcare providers and staff following pediatric appointments to confirm and clarify the interactions and behaviors we observed during appointments. Through these conversations, we were able to understand their thought process and mindsets during the appointment and learn any contextual information about the relationship between the provider and family that might have contributed to the conversation dynamic.

Findings
We analyzed 800+ data points from observations and interviews, 36 survey responses from participating parents/caregivers, and a variety of collected vaccine communication materials. Across the participating clinics, we observed patterns in how vaccine conversations were conducted, what materials were used, and which characteristics facilitated or created barriers to the vaccine conversations.
Clinical Vaccine Communication Flow

HCP Roles: Physicians/APPs tended to be the primary initiators of vaccine conversation. A distributed team involving receptionists, nurses, and medical assistants helped build trust with P/Cs before and after their interactions with their physician/APP. All play a critical role in sharing important vaccine safety information with families.
Conversation Duration and Timing: P/Cs without vaccine concerns had short vaccine conversations, ranging from 30 seconds to 3 minutes. For P/Cs with concerns, physicians/APPs spent more time addressing their questions, which extended the conversation time to 10-20 minutes. Vaccine conversations typically occurred during the middle or end of an appointment.
Communication Dynamic
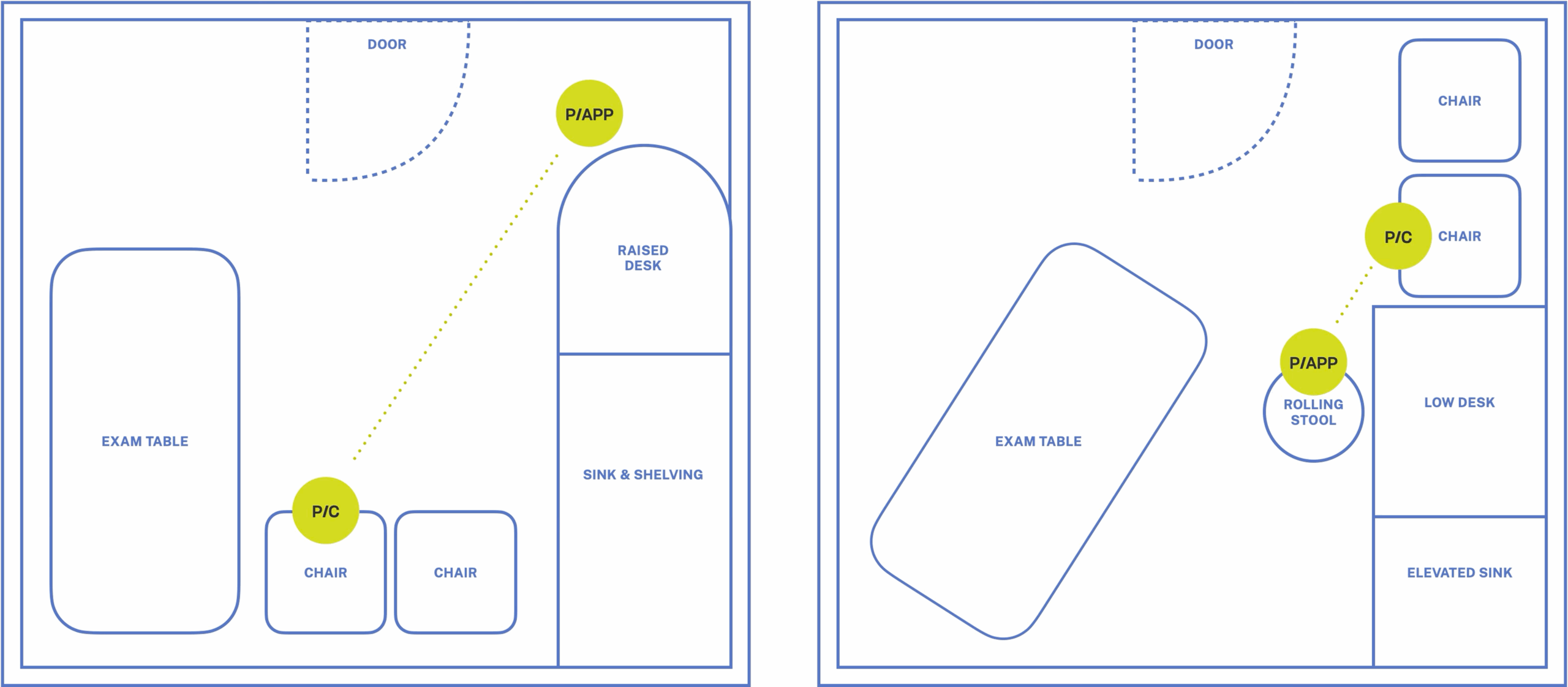
Provider Positioning
Two pediatric exam room floor plans illustrate the distance a provider and parent were positioned relative to each other.
Communication Needs and Preferences
Observed Archetypes and Communication Dynamics
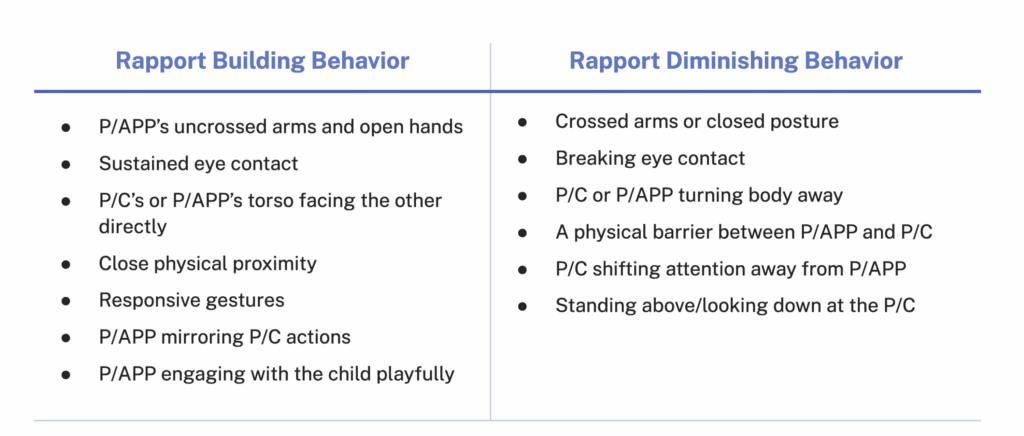
Key to our analysis is the finding that the communication dynamic between P/Cs and HCPs is shaped by the interaction of mindsets, communication needs, styles, and approaches. Based on our analysis, we generated eight recurrent archetypes for P/Cs and HCPs. These archetypes reflect a sizable subset of observed HCPs and P/Cs but are not exhaustive of all possible archetypes. HCPs and P/Cs may also shift from one archetype to another depending on the circumstance.
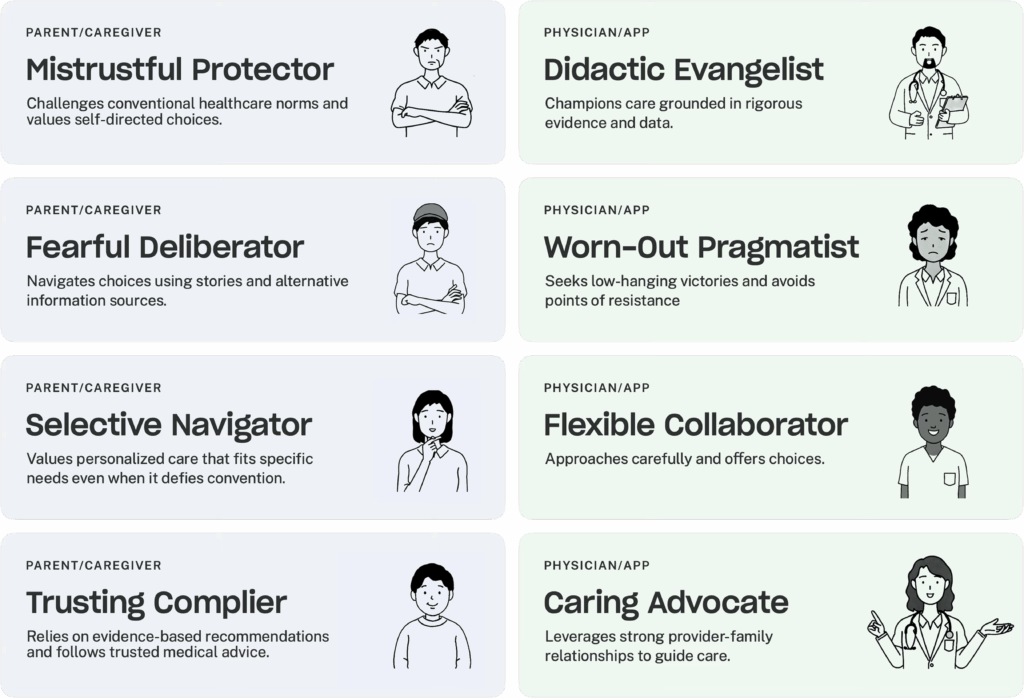
Using these eight HCP and P/C archetypes, we developed a series of pairs based on dynamics we witnessed across several appointment observations.
For example, one communication dynamic is a parent who is a Mistrustful Protector and a provider who is a Didactic Evangelist. Didactic Evangelists who are eager to correct misinformation risk alienating Mistrustful Protectors by ignoring their underlying concerns. This dismissive approach makes Mistrustful Protectors feel judged and unheard, which strengthens their vaccine resistance and damages the P/C-physician/APP relationship.
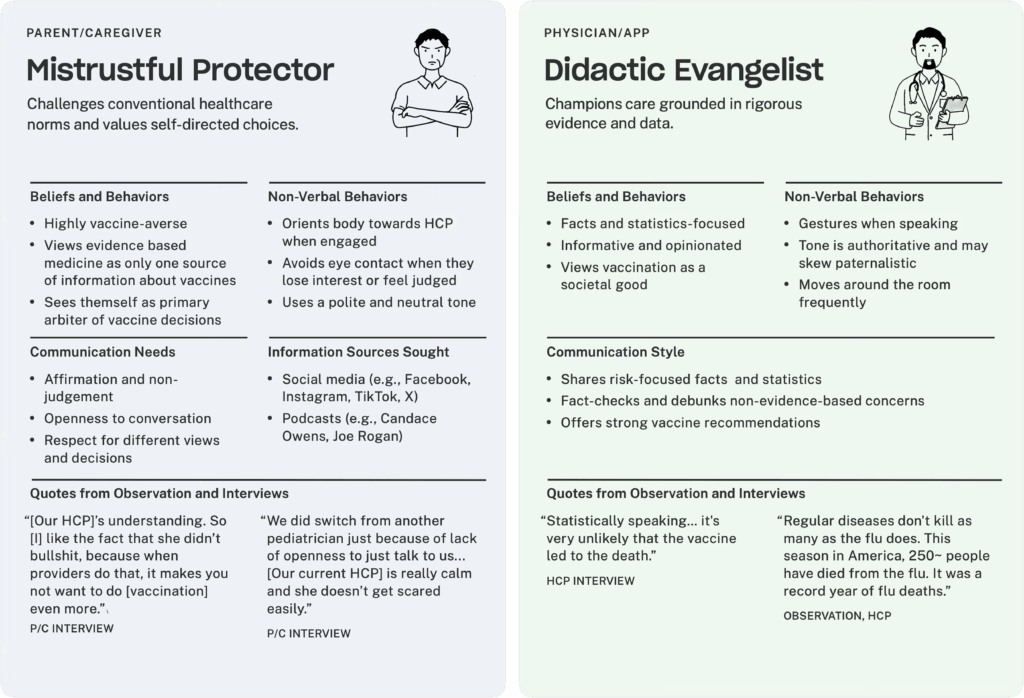
I don't like how pushy the conversation was. I don't like getting cut off. If you don’t answer my vaccine concern and just continue to push the vaccine instead of answering my questions, it’s very frustrating.”
—Caregiver
Our findings were combined with RTI’s parallel work streams to create a full 150+ page report, Improving Pediatric Vaccine Safety Communication. This report will be used by the CDC to improve communication about vaccine safety for children and identify recommendations to improve future vaccine communication products.
At the culmination of this project, we also co-facilitated a concept brainstorm workshop with RTI and the CDC to develop vaccine safety communication ideas for future exploration.
We invite partners to join us in the next phase of this work to create practical tools and training that strengthen vaccine communication with families. We’re looking for partners to co-develop and pilot:
Interested in collaborating? Contact us at info@publicpolicylab.org
PPL is a tax-exempt 501(c)(3)
nonprofit organization.
info@publicpolicylab.org
+1 646 535 6535
20 Jay Street, Suite 203
Brooklyn, NY 11201
We'd love to hear more. Send us a note and we'll be in touch.
We are accepting applications for a Graduate Summer Intern until February 23, 2026.
We're currently seeking applications for a Graduate Summer Intern. If interested, learn more about the role here.
To hear about future job announcements, follow us on Instagram, Twitter, Threads, and LinkedIn or subscribe to our newsletter.
Enter your email below to subscribe to our occasional newsletter.
Wondering what you’ve missed?
Check out our
The Public Policy Lab is a tax-exempt
501(c)(3) nonprofit organization.
Donate now to support our work; your
gift is tax-deductible as allowed by law.